Chapter 1 - Chemical Reactions and Equations (Ncert Solutions)
Ultimate NCERT Solutions for Chapter 1 Chemical Reactions and Equations
Updated Solution 2024-2025 Updated Solution 2024-2025
NCERT Solutions for Class 10 Science, Chapter 1 Chemical Reactions and Equations
(Question/Answers, Activity & Projects)
Chapter 1 Chemical Reactions and Equations
Activity 1.1
CAUTION: This activity needs the teacher’s assistance. It would be better if students wear suitable eyeglasses.
- Clean a magnesium ribbon, about 3-4 cm long by rubbing it with sand paper.
- Hold it with a pair of tongs. Burn it using a spirit lamp or a burner and collect the ash so formed in a watch- glass as shown in Fig. 1.1. Burn the magnesium ribbon keeping it away as far as possible from your eyes.

Fig 1.1. burning of magnesium ribbon in air and collection of magnesium oxide in watch glass
Q 1. What do you observe?
Ans 1: “When we burn a magnesium ribbon, it produces a bright white flame and forms a white powder known as magnesium oxide. This happens because magnesium reacts with oxygen from the air during burning.”
2Mg (s) + → 2MgO (s)
Activity 1.2
- Take lead nitrate solution in a test tube.
- Add potassium iodide solution to this.
Q 1. What do you observe?
Answer 1: When we mix the two substances, a chemical reaction occurs as follows:
Pb(NO₃)₂(aq) + 2KI(aq) → 2KNO₃(aq) + PbI₂(s)↓
In this reaction,
- Lead nitrate (Pb(NO₃)₂) reacts with potassium iodide (KI).
- This results in the formation of potassium nitrate (KNO₃), which remains dissolved in the solution, and lead iodide (PbI₂), which forms a bright yellow precipitate.
This is a double displacement and precipitation reaction, as an insoluble solid (PbI₂) is formed.
Activity 1.3
- Take a few zinc granules in a conical flask or a test tube.
- Add dilute hydrochloric acid or Sulphuric acid to this (Fig. 1.2).
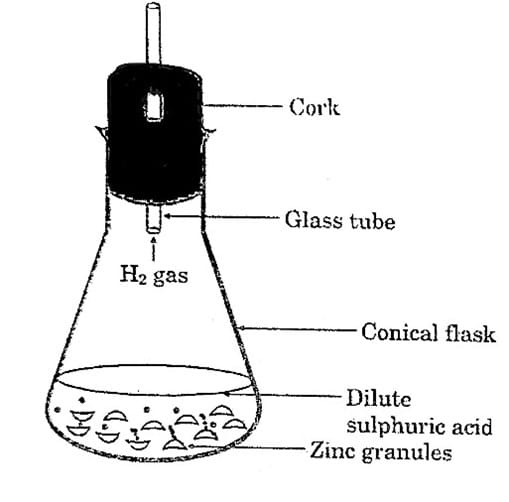
Fig. 1.2. Formation of hydrogen gas by the action of dilute Sulphuric acid on zinc
CAUTION: Handle the acid with care.
Q 1. Do you observe anything happening around the zinc granules?
Answer 1: Yes, as the reaction proceeds, the zinc granules gradually become smaller, and hydrogen gas is released.
Q 2. Touch the conical flask or test tube. Is there any change in its temperature?
Answer 2: Yes, the temperature has increased slightly.
Note: From the three activities above, we can conclude that the following observations indicate a chemical reaction:
- A change in state
- The release of gas
- A change in color
- A change in temperature
QUESTIONS
Q 1. Why should a magnesium ribbon be cleaned before burning in air?
Answer 1: Magnesium ribbon should be cleaned to remove any dust or oxide layer from its surface. This ensures that the metal directly contacts the air, allowing it to burn more easily and react fully with oxygen.
Q 2. Write the balanced equation for the following chemical reactions:
- Hydrogen + Chlorine→ Hydrogen chloride
- Barium chloride + Aluminum Sulphate → Barium sulphate + Aluminum chloride
- Sodium + Water → Sodium hydroxide+ Hydrogen
Answer 2:
- Hydrogen and chlorine react to form hydrogen chloride: H₂ + Cl₂ → 2HCl
- Barium chloride and aluminum sulfate react to form barium sulfate and aluminum chloride:3BaCl₂ + Al₂(SO₄)₃ → 3BaSO₄ + 2AlCl₃
- Sodium reacts with water to produce sodium hydroxide and hydrogen gas: 2Na + 2H₂O → 2NaOH + H₂↑
Q 3. Write a balanced chemical equation with state symbols for the following reactions:
- Solution of barium chloride and sodium sulphate in water react to give insoluble barium sulphate and the solution of sodium chloride.
- Sodium hydroxide solution (in water) reacts with hydrochloric acid solution (in water) to produce sodium chloride solution and water.
Ans 3:
(I): Reaction of barium chloride with sodium sulfate:
When solutions of barium chloride and sodium sulfate in water are mixed, they react to form an insoluble precipitate of barium sulfate and a solution of sodium chloride.
Balanced Equation:
BaCl₂(aq) + Na₂SO₄(aq) → BaSO₄(s)↓ + 2NaCl(aq)
(II): Reaction of sodium hydroxide with hydrochloric acid:
A solution of sodium hydroxide in water reacts with hydrochloric acid in water, producing a solution of sodium chloride and water.
Balanced Equation:
NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l)
Activity 1.4
- Take a small amount of calcium oxide or quick lime in a beaker.
- Slowly add water to this.
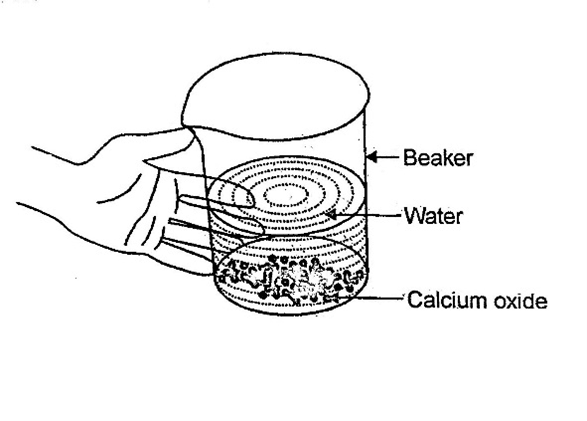
Fig. 1.3. Formation of slaked lime by the reaction of calcium oxide with water
- Touch the beaker as shown in Fig. 1.3.
Q 1. Do you feel any change in temperature?
Answer 1: Yes, the temperature has significantly increased due to the following reaction:
CaO + H₂O → Ca(OH)₂ + Heat
In this reaction, calcium oxide (CaO) reacts with water (H₂O) to produce calcium hydroxide Ca(OH)₂ and release heat. This is an exothermic reaction.
Activity 1.5
- Take about 2 g ferrous sulphate crystals in a dry boiling tube.
- Note the colour of the ferrous sulphate crystals.
- Heat the boiling tube over the flame of a burner or spirit lamp as shown in Fig. 1.4.

Fig. 1.4. Correct way of heating the test tube containing crystals of ferrous sulphate and of smelling the odor
Q 1: Observe the colour of the crystals after heating.
Answer 1:
Before heating, ferrous sulfate crystals are green due to the presence of water of crystallization (FeSO₄·7H₂O). Upon heating, the crystals first lose water and become white (anhydrous FeSO₄). Further heating causes decomposition, forming reddish-brown ferric oxide (Fe₂O₃) and releasing sulfur dioxide (SO₂) and sulfur trioxide (SO₃) gases, both of which have a pungent odour.
Observation:
Green FeSO₄·7H₂O → White FeSO₄ → Reddish-brown Fe₂O₃ + SO₂ + SO₃ (gases)
Balanced Chemical Equation:
2FeSO₄ (s) → Fe₂O₃ (s) + SO₂ (g) ↑ + SO₃ (g) ↑
Activity 1.6
Take about 2 g lead nitrate powder in a boiling tube.
- Hold the boiling tube with a pair of tongs and heat it over a flame, as shown in Fig. 1.5.

FIG 1.5. heating of lead nitrate and emission of nitrogen dioxide
Q 1. What do you observe? Note down the change, if any.
Answer 1: Observation: The emission of brown fumes is observed. These fumes are identified as nitrogen dioxide (NO₂). The chemical reaction occurring is as follows:
Reaction:
2Pb (NO₃) ₂ → 2PbO + 4NO₂ + O₂
Activity 1.7
- Take a plastic mug. Drill two holes at its base and fit rubber stoppers in these holes. Insert carbon electrodes in these rubber stoppers as shown in Fig. 1.6.
- Connect these electrodes to a 6-volt battery.
- Fill the mug with water such that the electrodes are immersed. Add a few drops of dilute Sulphuric acid to the water.
- Take two test tubes filled with water and invert them over the two carbon electrodes.
- Switch on the current and leave the apparatus undisturbed for some time.
- You will observe the formation of bubbles at both the electrodes. These bubbles displace water in the test tubes.
Q 1. Is the volume of the gas collecting the same in both the test tubes?
Answer 1: No, the volumes of gas collected in each test tube are different.
- Once the test tubes are filled with the respective gases, remove them carefully.
- Test these gases one by one by bringing a burning candle close to the mouth of the test tubes.
CAUTION: This step must be performed carefully by the teacher.

Fig. 1.6. Electrolysis of water
Q 2. What happens in each case?
Ans 2: “The gas volume in one test tube is twice as large as the volume in the other test tube.”
Q 3. Which gas is present in each test tube?
Ans 3: “In one test tube, hydrogen gas is contained, while oxygen gas is stored in a separate test tube.”
Activity 1.8
- Take about 2 g silver chloride in a China dish.
Q 1. What is its colour?
Ans 1: It is white in color.
- Place this China dish in sunlight for some time (Fig. 1.7).

Fig. 1.7. Silver chloride turns grey in sunlight to form silver metal
Q 1: Observe the colour of the silver chloride after some time.
Answer 1:
Observation: Initially, silver chloride appears white, but after exposure to sunlight, it gradually changes to a grey color. This change occurs because sunlight causes the silver chloride to break down into silver and chlorine gas. The chemical reaction is as follows:
2AgCl (in sunlight) → 2Ag + Cl₂
Carry out the following activity
Q 1. Take about 2 g barium hydroxide in a test tube. Add 1 g of ammonium chloride and mix with the help of a glass rod. Touch the bottom of the test tube with your palm. What do you feel? Is this an exothermic or endothermic reaction?
Ans 1:
When you mix barium hydroxide (Ba(OH)₂·8H₂O) and ammonium chloride (NH₄Cl) in the test tube, the bottom of the test tube feels cold when touched. This indicates that the reaction is endothermic.
Explanation:
The chemical reaction between barium hydroxide octahydrate and ammonium chloride is as follows:
Ba(OH)₂·8H₂O (s) + 2NH₄Cl (s) → BaCl₂ (aq) + 2NH₃ (g) + 10H₂O (l)
This reaction absorbs heat from the surroundings, leading to a noticeable cooling effect. Hence, it is classified as an endothermic reaction, which means it requires energy input in the form of heat from the environment.
QUESTIONS
Q 1. A solution of a substance X is used for white- washing.
- Name the substance X and write its formula.
- Write the reaction of the substance X named in (i) above with water.
Ans 1: (i) The substance X is Calcium oxide, commonly known as quicklime. Its chemical formula is CaO.
ii) Reaction of Calcium Oxide (CaO) with Water:
When calcium oxide reacts with water, it forms calcium hydroxide (Ca(OH)₂), releasing a large amount of heat. This reaction is called slaking of lime.
Chemical Equation:
CaO (s) + H₂O (l) → Ca(OH)₂ (aq) + Heat
Q 2. Why is the amount of gas collected in one of the test tubes in Activity 1.7, double of the amount collected in the other? Name this gas.
Ans 2: “The gas in one test tube is twice the volume of the gas in the other because it is hydrogen, while the other contains oxygen. Both gases are produced through the electrolysis of water, and the volume of hydrogen is twice that of oxygen.”
ACTIVITY 1.9
- Take three iron nails and clean them by rubbing with sand paper.
- Take two test tubes marked as (A) and (B). In each test tube, take about 10 ml copper sulphate solution.
- Tie two iron nails with a thread and immerse them carefully in the copper sulphate solution in test tube B for about 20 minutes [Fig. 1.8 (a)]. Keep one iron nail aside for comparison.
- After 20 minutes, take out the iron nails from the copper sulphate solution.
- Compare the intensity of the blue colour of copper sulphate solutions in test tubes (A) and (B) [Fig. 1.8 (b)].
- Also, compare the colour of the iron nails dipped in the copper sulphate solution with the one kept aside [Fig. 1.8 (b)].

Fig 1.8 (a) iron nails dipped in copper sulphate solution

Fig. 1.8. (b) Iron nails and copper sulphate solutions compared before and after the experiment
On the basis of activity 1.9, give the answers of the following questions:
Q 1. What is the colour of copper sulphate solution in test tube A?
Ans 1: The solution is blue in color.
Q 2. What is the colour of copper sulphate solution in test tube B?
Ans 2: The copper sulfate solution in test tube B has lost its blue color and appears faded.
Q 3. Why does the blue colour of copper sulphate solution fade?
Ans 3: The blue color of copper sulfate solution fades because copper ions are displaced in the reaction. When copper sulfate is exposed to certain conditions, such as heating or the presence of another substance that can react with copper, the copper ions are replaced, leading to a loss of the characteristic blue color.
Q 4. What is the colour of iron nail in test tube B?
Ans 4: The iron nail turns brown in color.
Q 5. Why does the iron nail become brownish in colour?
Ans 5: The brownish colour on an iron nail occurs because copper gets deposited on its surface. This happens when iron (Fe) reacts with copper(II) sulfate (CuSO₄). As a result, iron sulfate (FeSO₄) forms in the solution and copper (Cu) gets deposited on the nail.
Chemical reaction:
CuSO₄ + Fe → FeSO₄ + Cu
This is a displacement reaction, where a more reactive metal (iron) displaces a less reactive metal (copper) from its salt solution.
Activity 1.10
- Take about 3 ml of sodium sulphate solution in a test tube:
- In another test tube, take about 3 ml of barium chloride solution.
- Mix the two solutions (Fig. 1.9).

Fig. 1.9 Formation of barium sulphate and sodium chloride
Q 1. What do you observe?
Ans 1: A white solid is formed, which does not dissolve in water.
Chemical Reaction:
Na₂SO₄ + BaCl₂ → 2NaCl + BaSO₄
Note: BaSO₄ (barium sulfate) is the white precipitate formed in this double displacement reaction.
Activity 1.11
- Heat a China dish containing about 1 g copper powder (Fig. 1.10).

Fig. 1.10. Oxidation of copper-to-copper oxide
Q 1: What do you observe?
Ans 1: When copper powder is exposed to oxygen, its surface gets coated with black copper(II) oxide. This occurs due to a chemical reaction between the copper and oxygen, especially when heat is applied.
The reaction can be represented as: 2Cu + O₂ (Heat) → 2CuO
QUESTIONS
Q 1. Why does the colour of copper sulphate solution change when an iron nail is dipped in it?
Ans 1:
When an iron nail is dipped into a copper sulphate solution, the colour of the solution changes due to a displacement reaction. Iron is more reactive than copper and displaces copper from copper sulphate, forming iron sulphate (FeSO₄) and copper (Cu).
Chemical Equation:
CuSO₄ (aq) + Fe (s) → FeSO₄ (aq) + Cu (s)
As a result, the blue colour of the copper sulphate solution fades, and a reddish-brown deposit of copper forms on the iron nail.
Q2. Give an example of a double displacement reaction other than the one given in Activity 1.10.
Ans 2: An example of a double displacement reaction is when lead(II) nitrate reacts with potassium iodide. This reaction produces potassium nitrate and lead(II) iodide (a yellow precipitate).
Balanced Chemical Equation:
Pb(NO₃)₂ + 2KI → 2KNO₃ + PbI₂
Q 3. Identify the substances that are oxidised and the substances that are reduced in the following reactions:
(i) 4Na(s) + O₂(g) → 2Na₂O(s)
(ii) CuO(s) + H₂(g) → Cu(s) + H₂O(l)
Ans 3:
(i) In this reaction, sodium (Na) is oxidized to form sodium oxide (Na₂O), meaning it loses electrons.
(ii) In this reaction, copper oxide (CuO) is reduced to copper (Cu) by gaining electrons, while hydrogen (H₂) is oxidized to form water (H₂O) by losing electrons.
EXERCISES
Q1. Which of the statements about the reaction below are incorrect?
2PbO(s) + C(s) → 2Pb(s) + CO₂(g)
(a) Lead is getting reduced.
(b) Carbon dioxide is getting oxidised.
(c) Carbon is getting oxidised.
(d) Lead oxide is getting reduced.
(i) a and b
(ii) a and c
(iii) a, b and c
(iv) all
Ans 1: Let’s analyze each statement:
(a) Lead is getting reduced:
✅ Correct. Lead oxide (PbO) is reduced to lead (Pb), as it loses oxygen.
(b) Carbon dioxide is getting oxidised:
❌ Incorrect. Carbon dioxide (CO₂) is a product of the reaction and carbon is already in its highest oxidation state (+4), so it cannot be further oxidized.
(c) Carbon is getting oxidised:
✅ Correct. Carbon (C) is oxidized to carbon dioxide (CO₂) as it gains oxygen.
(d) Lead oxide is getting reduced:
✅ Correct. Lead oxide (PbO) loses oxygen to form lead (Pb), meaning it is reduced.
📝 The incorrect statement is (b), so the correct answer is:
Ans: (i) a and b
Q2. Reaction: Fe₂O₃ + 2Al → Al₂O₃ + 2Fe
The above reaction is an example of a:
(a) Combination reaction
(b) Double displacement reaction
(c) Decomposition reaction
(d) Displacement reaction
Answer: (d) Displacement reaction
Explanation: Aluminum (Al) displaces iron (Fe) from iron(III) oxide (Fe₂O₃) to form aluminum oxide (Al₂O₃) and free iron (Fe). This is a classic displacement reaction.
Q3. What happens when dilute hydrochloric acid is added to iron filings?
(a) Hydrogen gas and iron chloride are produced.
(b) Chlorine gas and iron hydroxide are produced.
(c) No reaction takes place.
(d) Iron salt and water are produced.
Answer: (a)
Explanation: Iron (Fe) reacts with hydrochloric acid (HCl) to form iron(II) chloride (FeCl₂) and hydrogen gas (H₂).
Equation:
Fe + 2HCl → FeCl₂ + H₂↑
Q 4. What is a balanced chemical equation? Why should chemical equations be balanced?
Ans 4: A chemical equation is considered balanced when the number of atoms of each element is the same on both sides. Balancing chemical equations is important because it accurately represents the number of reactants and products involved, providing a true reflection of the chemical reaction.
Q 5. Translate the following statements into chemical equations and then balance them.
(a) Hydrogen gas combines with nitrogen to form ammonia.
(b) Hydrogen sulphide gas burns in air to give water and Sulphur dioxide.
(c) Barium chloride reacts with aluminum sulphate to give aluminum chloride and a precipitate of barium sulphate.
(d) Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
Ans 5:
(a) Hydrogen gas combines with nitrogen to form ammonia.
Chemical equation:
N₂(g) + H₂(g) → NH₃(g)Balanced equation:
N₂(g) + 3H₂(g) → 2NH₃(g)
(b) Hydrogen sulphide gas burns in air to give water and sulphur dioxide.
Chemical equation:
H₂S(g) + O₂(g) → H₂O(l) + SO₂(g)Balanced equation:
2H₂S(g) + 3O₂(g) → 2H₂O(l) + 2SO₂(g)
(c) Barium chloride reacts with aluminum sulphate to give aluminum chloride and a precipitate of barium sulphate.
Chemical equation:
BaCl₂(aq) + Al₂(SO₄)₃(aq) → AlCl₃(aq) + BaSO₄(s)Balanced equation:
3BaCl₂(aq) + Al₂(SO₄)₃(aq) → 2AlCl₃(aq) + 3BaSO₄(s)
(d) Potassium reacts with water to give potassium hydroxide and hydrogen gas.
Chemical equation:
K(s) + H₂O(l) → KOH(aq) + H₂(g)Balanced equation:
2K(s) + 2H₂O(l) → 2KOH(aq) + H₂(g)
Q6. Balance the following chemical equations:
(a) HNO₃ + Ca(OH)₂ → Ca(NO₃)₂ + H₂O
Ans a): Balanced Equation: 2HNO₃ + Ca(OH)₂ → Ca(NO₃)₂ + 2H₂O
(b) NaOH + H₂SO₄ → Na₂SO₄ + H₂O
Ans b): Balanced Equation: 2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂O
(c) NaCl + AgNO₃ → AgCl + NaNO₃
Ans c): Balanced Equation: Already balanced.
(d) BaCl₂ + H₂SO₄ → BaSO₄ + HCl
Ans d): Balanced Equation: BaCl₂ + H₂SO₄ → BaSO₄ + 2HCl
Q7. Write balanced chemical equations:
(a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water
Ans a): Ca(OH)₂(aq) + CO₂(g) → CaCO₃(s) + H₂O(l)
(b) Zinc + Silver nitrate → Zinc nitrate + Silver
Ans b): Zn(s) + 2AgNO₃(aq) → Zn(NO₃)₂(aq) + 2Ag(s)
(c) Aluminum + Copper(II) chloride → Aluminum chloride + Copper
Ans c): 2Al(s) + 3CuCl₂(aq) → 2AlCl₃(aq) + 3Cu(s)
(d) Barium chloride + Potassium sulphate → Barium sulphate + Potassium chloride
Ans d): BaCl₂(aq) + K₂SO₄(aq) → BaSO₄(s) + 2KCl(aq)
Q 8. Write the balanced chemical equation for the following and identify the type of reaction in each case.
(a) Potassium bromide(aq) +Barium iodide (aq) → Potassium iodide(aq) + Barium bromide(s)
Ans 8: (a) Potassium bromide(aq) + Barium iodide(aq) → Potassium iodide(aq) + Barium bromide(s)
Balanced Equation:
2KBr(aq) + BaI₂(aq) → 2KI(aq) + BaBr₂(s)
Type of Reaction: This is a Double Displacement (or Double Replacement) reaction, where the cations and anions of two compounds exchange places, forming a precipitate (BaBr₂).
Ans (b): Zinc carbonate(s) → Zinc oxide(s) + Carbon dioxide(g)
Balanced Equation:
ZnCO₃(s) → ZnO(s) + CO₂(g)
Type of Reaction: This is a Decomposition reaction, where a single compound (zinc carbonate) breaks down into two products (zinc oxide and carbon dioxide) upon heating.
Ans (c): Hydrogen (g) + Chlorine (g) → Hydrogen chloride (g)
Balanced Equation:
H₂ (g) + Cl₂ (g) → 2 HCl (g)
Type of Reaction: This is a Synthesis (or Combination) reaction, where two elements (hydrogen and chlorine) combine to form a compound (hydrogen chloride).
Ans (d): Magnesium(s) + Hydrochloric acid(aq) → Magnesium chloride(aq) + Hydrogen(g)
Balanced Equation:
Mg(s) + 2 HCl(aq) → MgCl₂(aq) + H₂(g)
Type of Reaction: This is a Single Displacement (or Single Replacement) reaction, where magnesium displaces hydrogen from hydrochloric acid, producing magnesium chloride and hydrogen gas.
Q 9. What does one mean by exothermic and endothermic reactions? Give examples.
Ans 9: Exothermic Reactions:
Reactions that release energy (usually heat) to the surroundings.
Examples:
2H₂ + O₂ → 2H₂O + Heat
2Al + Fe₂O₃ → 2Fe + Al₂O₃ + Heat (Thermite reaction)
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + Energy (Respiration)
Endothermic Reactions:
Reactions that absorb energy (heat) from the surroundings.
Examples:
6CO₂ + 6H₂O + Energy (sunlight) → C₆H₁₂O₆ + 6O₂ (Photosynthesis)
Dissolution of ammonium nitrate in water
Chapter 1 Chemical Reactions and Equations
Updated Solution 2024-2025
Q 10. Why is respiration considered an exothermic reaction? Explain.
Ans 10: Respiration is considered an exothermic reaction because it releases energy during the breakdown of food (usually glucose) in the presence of oxygen. In this process, glucose (C₆H₁₂O₆) is oxidized, producing carbon dioxide (CO₂) and water (H₂O) as byproducts, along with the release of energy. The energy released is used by organisms for various functions like movement, growth, and maintaining body temperature.
The chemical equation for aerobic respiration is:
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + Energy
Since energy is released in the form of ATP (adenosine triphosphate), respiration is classified as an exothermic reaction, meaning it gives off heat or energy to the surroundings.
Q 11. Why are decomposition reactions called the opposite of combination reactions? Write equations for these reactions.
Ans 11: Decomposition Reactions: In decomposition reactions, a single compound breaks down into two or more simpler substances, either elements or smaller compounds. Example of Decomposition Reaction:
CaCO₃ (s) → CaO (s) + CO₂ (g)
This is a decomposition reaction because one compound (calcium carbonate) breaks into two products (calcium oxide and carbon dioxide).
- Combination Reactions: In combination reactions, two or more substances combine to form a single new substance.
Example of Combination Reaction:
CaO (s) + CO₂ (g) → CaCO₃ (s)
This is a combination reaction because two substances (calcium oxide and carbon dioxide) combine to form a single new compound (calcium carbonate).
Since decomposition reactions break compounds down (opposite of combination), they are considered the reverse of combination reactions.
Q 12. Write one equation each for decomposition reactions where energy is supplied in the form of heat, light or electricity.
Ans 12:
1.)Energy supplied in the form of heat:
CaCO₃ → CaO + CO₂
(Calcium carbonate decomposes into calcium oxide and carbon dioxide upon heating.)
2.) Energy supplied in the form of light:
2AgCl (s) → 2Ag (s) + Cl₂ (g) (in sunlight)
(Silver chloride decomposes into silver and chlorine gas under sunlight.)
3.) Energy supplied in the form of electricity:
2H₂O → 2H₂ + O₂
(Water decomposes into hydrogen and oxygen gas through electrolysis.)
Q 13. What is the difference between displacement and double displacement reactions? Write equations for these reactions.
Ans 13: A displacement reaction occurs when a more reactive element displaces a less reactive element from a compound. For example, in the reaction below, zinc (Zn), being more reactive, displaces copper (Cu) from copper sulfate (CuSO₄):
CuSO₄ + Zn → ZnSO₄ + Cu
In a double displacement reaction, two ionic compounds react by exchanging their ions to form new products. For example, when sodium sulfate (Na₂SO₄) reacts with barium chloride (BaCl₂), the ions swap, producing barium sulfate (BaSO₄) and sodium chloride (NaCl):
Na₂SO₄(aq) + BaCl₂(aq) → BaSO₄(s) + 2NaCl(aq)
Q 14. In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the reaction involved.
Ans 14: When copper is added to a silver nitrate solution, it displaces the silver because copper is more reactive than silver. The reaction is as follows:
2 AgNO₃ + Cu → Cu(NO₃)₂ + 2 Ag
In this reaction:
- Copper (Cu) displaces silver (Ag) from silver nitrate (AgNO₃) because copper is more reactive than silver.
- Copper nitrate (Cu(NO₃)₂) is formed, and silver metal (Ag) is displaced from the solution.
Q 15. What do you mean by a precipitation reaction? Explain by giving examples.
Answer 15: A precipitation reaction occurs when two aqueous solutions react to form an insoluble solid, called a precipitate. This solid separates from the solution during the reaction.
Examples:
1. When sodium sulfate (Na₂SO₄) reacts with barium chloride (BaCl₂), a white precipitate of barium sulfate (BaSO₄) forms:
Na₂SO₄(aq) + BaCl₂(aq) → BaSO₄(s) + 2NaCl(aq)
2. In the reaction between lead(II) nitrate (Pb(NO₃)₂) and potassium iodide (KI), lead(II) iodide (PbI₂) precipitates:
Pb(NO₃)₂(aq) + 2KI(aq) → 2KNO₃(aq) + PbI₂(s)
In both cases, the solid formed is the precipitate, and it separates from the liquid solution.
Q 16. Explain the following terms of gain or loss of oxygen with two examples each.
(a) Oxidation (b) Reduction
Ans 16: (a) Oxidation: Oxidation refers to the chemical reactions in which an element gains oxygen.
Examples of Oxidation:
- When copper (Cu) reacts with oxygen (O₂), copper oxide (CuO) is formed:
2Cu + O₂ → 2CuO - When hydrogen (H₂) combines with oxygen (O₂), water (H₂O) is produced:
2H₂ + O₂ → 2H₂O
(b) Reduction: Reduction involves the chemical reactions where an element loses oxygen.
Examples of Reduction:
- When zinc oxide (ZnO) reacts with carbon (C), zinc (Zn) is produced:
ZnO + C → Zn + CO - When copper (CuO) reacts with hydrogen (H₂), copper (Cu) and water (H₂O) are formed:
CuO + H₂ → Cu + H₂O
Q 17. A shiny brown coloured element X’ on heating in air becomes black in colour. Name the element X and the black-coloured compound formed.
Ans 17: The element is copper (Cu). When copper is heated in the presence of air, it reacts with oxygen to form copper oxide (CuO), which gives it a black color.
The chemical reaction is: 2Cu + O₂ → 2CuO
Q 18. Why do we apply paint on iron articles?
Ans 18: We apply paint on iron articles for the following reasons:
- Protection from Rust: Paint acts as a barrier, preventing moisture and air from reaching the metal surface, reducing rust formation.
- Durability: It enhances the lifespan of iron by shielding it from environmental factors like rain, salt, and chemicals.
- Aesthetic Appeal: Paint improves the appearance of iron articles, giving them a smooth, clean, and colorful finish.
- Prevention of Corrosion: The protective layer of paint prevents the iron from corroding, especially in humid or coastal areas.
- Maintenance of Strength: Paint helps preserve the structural integrity of iron by preventing the weakening effect of rust.
Q 19. Oil and fat containing food items are flushed with nitrogen. Why?
Ans 19: Oil and fat-containing food items are flushed with nitrogen for the following reasons:
- Prevents Oxidation: Nitrogen displaces oxygen, preventing oxidation that could cause rancidity.
- Maintains Freshness: Helps preserve the flavor and texture of the food.
- Extends Shelf Life: Reduces the growth of aerobic bacteria and mold.
- Prevents Spoilage: Keeps the food safe from spoilage due to air exposure.
Q 20. Explain the following terms with one example each:
(a) Corrosion
Ans (a) Corrosion: Corrosion is the gradual destruction of materials, usually metals, by chemical reactions with environmental elements like oxygen, water, or acids. The most common form of corrosion is rusting, where iron reacts with oxygen and moisture to form iron oxide.
- Example: Rusting of iron. When iron is exposed to moisture and air, it reacts to form rust (iron oxide), weakening the metal.
(b) Rancidity
(b) Rancidity: Rancidity refers to the process by which fats and oils degrade, often due to oxidation or microbial action, leading to unpleasant odors or flavors. It commonly happens in food products containing unsaturated fats.
- Example: The rancid smell of old cooking oil. When oil is exposed to air over time, it oxidizes and develops a foul odor, indicating that it has gone rancid.
Group Activity
Perform the following activity.
- Take four beakers and label them as A, B, C and D.
- Put 25 mL of water in A, B and C beakers and copper sulphate solution in beaker D.
- Measure and record the temperature of each liquid contained in the beakers above.
- Add two spatulas of potassium sulphate, ammonium nitrate, anhydrous copper
- sulphate and fine iron fillings to beakers A, B, C and D respectively and stir.
- Finally measure and record the temperature of each of the mixture above.
Find out which reactions are exothermic and which ones are endothermic in nature
Ans: [Note: students do yourself with the help of your teachers in laboratory]
Apply this method
This experiment involves adding various substances to liquids in beakers and measuring the temperature changes to determine whether the reactions are exothermic or endothermic. Here’s a step-by-step outline of how to perform the activity and interpret the results:
Materials Needed:
- 4 beakers (labeled A, B, C, and D)
- Water (25 mL each for beakers A, B, and C)
- Copper sulphate solution (for beaker D)
- Potassium sulphate
- Ammonium nitrate
- Anhydrous copper sulphate
- Fine iron fillings
- Thermometer
- Stirring rod or spatula
Procedure:
1.Label the Beakers:
- Label four beakers as A, B, C, and D.
2. Add Liquids:
- Fill beakers A, B, and C with 25 mL of water each.
- Fill beaker D with copper sulphate solution.
3. Measure Initial Temperature:
- Use a thermometer to measure and record the temperature of the liquid in each beaker before adding any solids.
4. Add Solid Substances:
- In beaker A, add two spatulas of potassium sulphate.
- In beaker B, add two spatulas of ammonium nitrate.
- In beaker C, add two spatulas of anhydrous copper sulphate.
- In beaker D, add two spatulas of fine iron fillings.
5. Stir the Mixtures:
- Stir each beaker well to ensure the substances are mixed properly.
6. Measure Final Temperature:
- After stirring, measure and record the temperature in each beaker again.
Observation and Analysis:
- Exothermic Reactions: If the temperature of the mixture increases, it indicates that heat is being released during the reaction. This is an exothermic reaction.
- Endothermic Reactions: If the temperature of the mixture decreases, it indicates that heat is being absorbed from the surroundings, which is characteristic of an endothermic reaction.
Expected Outcomes:
- Beaker A (Potassium Sulphate + Water): Potassium sulphate dissolving in water typically absorbs heat, so this may be an endothermic reaction.
- Beaker B (Ammonium Nitrate + Water): The dissolution of ammonium nitrate in water is generally an endothermic process, so the temperature will likely drop.
- Beaker C (Anhydrous Copper Sulphate + Water): When anhydrous copper sulphate reacts with water, it often results in heat release, making it an exothermic reaction.
- Beaker D (Iron Fillings + Copper Sulphate Solution): The reaction between iron fillings and copper sulphate is typically exothermic because it releases heat as iron displaces copper from the solution.
Conclusion:
1. After measuring the final temperatures and comparing them with the initial ones, you can classify the reactions as exothermic or endothermic based on the observed temperature changes:
- Exothermic reactions: If the temperature increases.
- Endothermic reactions: If the temperature decreases.
Chapter 1 Chemical Reactions and Equations
Updated Solution 2024-2025
This complete solution is prepared as per the latest syllabus of 2024-25. If you have any further queries, feel free to ask! 😊


